Dessa Sadovnick’s lonely quest to show that genes can play a major role in multiple sclerosis

A. Dessa Sadovnick. Photo: Paul Joseph
Dessa Sadovnick has devoted her career to chipping away at the conventional wisdom in her field.
The UBC professor of medical genetics and neurology has long been convinced that genes play a major role in conditions like multiple sclerosis and Alzheimer’s disease – a concept that seems self-evident today, 16 years after the decoding of the human genome. But when she first arrived at UBC as a resolute graduate student four decades ago, the notion was dismissed and derided.
Her PhD supervisor at UBC urged her to abandon her chosen dissertation topic after a journal article said genetics was irrelevant to MS. After earning her doctorate, she presented her findings about the heritability of MS at a conference, only to be interrupted by the organizer, who declared her thesis implausible, telling her to wrap it up in two minutes. Later, as a faculty member, she sought permission from the federal government to collect DNA samples from people with age-related dementia; her request, deemed a waste of time and money, was denied.
But she persisted, going on to create the largest MS biobank and database of its kind, with funding from the MS Society of Canada. Now, thanks to that collection of blood and extracted DNA, amassed over 20 years and stored in a freezer on UBC’s campus, she has been vindicated.
Ahead of the game
Dr. Sadovnick is a co-author of an article, published in the journal Neuron in June, describing a rare genetic mutation that appears to give a 70 per cent chance of developing the progressive form of MS.
The discovery was led by a younger colleague, Assistant Professor Carles Vilarino-Guell, who applied state-of-the-art genotyping technology to samples from Dr. Sadovnick’s biobank.
“There are still people today who don’t believe that familial forms of MS exist,” Dr. Vilarino-Guell says. “Dessa was very ahead of the game.”
Dr. Sadovnick remains guarded. Most scientists have come to accept that genes contribute to MS, but as bit players – making a person just slightly more vulnerable to more powerful environmental causes, such as diet, climate, behavior, viruses or microbes. The direct linking of one mutation to MS, even in just a small number of cases, makes a strong case that genes could be major players in who gets the disease, and who doesn’t.
“Acceptance of a new concept is not something that happens overnight, so there are probably going to be a lot of people saying it’s nonsense, because it’s so against what people tend to believe,” she says.
Study what you know

Eveyln Opal. Photo courtesy of MS Society of Canada
Dr. Sadovnick’s interest in MS was hardly academic. As a child in Montreal, she helped care for her mother’s close friend, Eveyln Opal, a co-founder of the MS Society of Canada who had been bedridden with the disease since her 20s.
Just before Dr. Sadovnick headed west to pursue her PhD in medical genetics at UBC, Opal suggested that she focus on MS.
“It’s what you know about,” Opal told her.
But her supervisors, upon learning of her intentions, were skeptical.
“Everybody said, ‘There’s nothing genetic about MS, we don’t know much about it, there’s no treatment,” she recalls. “People thought I was nuts. Back then, genetics was mostly focused on birth defects. Adult disorders were not thought of in terms of genetics. But I’m a sucker.”
She traveled the province, tracking down people with MS, especially those who also had relatives with the condition. Though she didn’t have the resources to do a well-controlled study, she found a surprising number of cases where it seemed to run in the family.
Those anecdotal cases were hardly proof, and were largely explained away by the fact that family members are usually exposed to the same set of similar environmental factors – diet, climate, behavior, viruses and microbes – that were presumed to be the causes of the disease.
A longshot request
But she soldiered on, earning her PhD on the genetics of MS. And that might have been the end of it. She took a research position at UBC working on neural tube defects – a “safe” topic for someone with a doctorate in medical genetics.
Then Dr. Sadovnick learned that UBC had recruited a prominent neurologist, Don Paty, to start an MS clinic. So she tracked him down at a public event to see if he would consider adding a geneticist – her, of course — to his team.
Not surprisingly, Dr. Paty was hesitant, bearing the same doubts as the rest of his colleagues. When someone in the audience asked if MS could be inherited, he firmly rejected the idea. But members of the audience kept asking about that, citing their own family histories.
“So when the question period was over,” she recalls, “Don turned to me and said, ‘OK, I guess you better look into it.’”
Gathering blood and data
Her persistence eventually won over the MS Society of Canada, which provided funds to create a Canada-wide collection of DNA from MS patients, along with as many relatives as they could get, and associated clinical and demographic information. The very name indicated a growing acceptance of Dr. Sadovnick’s hunch: the Canadian Collaborative Project on Genetic Susceptibility to Multiple Sclerosis.
Samples and data were collected from every MS clinic in the country, and over 20 years,the collection was updated and expanded. The wealth of detail, unmatched by any other MS biobank in the world, enabled Dr. Sadovnick to find more evidence for the role of genetics – finding, for example, that MS tends to be transmitted more from unaffected mothers than from unaffected fathers.
As the power of genetics permeated medicine, resistance to the heritability of MS began to break down. A few genes have been implicated in MS, appearing to slightly increase a person’s risk of getting the disease. The mutation with the strongest association carried a 6 per cent heightened risk compared to the general population.
Inter-generational frustration
But the elusiveness of a causative gene – similar to the gene for Huntington’s disease, or the one for early-onset Alzheimer’s disease – hindered efforts to explore MS’s genetic dimension.
Dr. Vilarino-Guell inherited Dr. Sadovnick’s frustration. Two years ago, a prominent MS scientist visited UBC and declared, in a room full of people, that Dr. Vilarino-Guell would never succeed in finding an MS gene.

Carles Vilarino-Guell. Photo: Paul Joseph
“It was the same thing that Dessa has been facing for 40 years,” he says. “I’ve only experienced two years of it, and it still burns me. We could be doing so much more if we could get funding, but the idea is still so unorthodox.”
Dr. Vilarino-Guell, like Dr. Sadovnick, was undeterred. Plus, he had access to state-of-the-art gene sequencing technology in the Djavad Mowafaghian Centre for Brain Health. But first, he had to figure out which of the 13,000 usable samples to analyze.
A process of elimination
First, he searched the database for families where four or more closely-related people had MS, and where the affected members had provided blood samples.
He ranked those remaining families based on the homogeneity of the affected family members’ disease – looking for families where they all had progressive MS, which causes a steady deterioration, or they all had relapsing-remitting MS, where symptoms come and go for several years.
In many cases, detailed clinical information was only available for one family member. But about 25 families fit the profile.
He then pulled their DNA samples from a freezer in the brain health centre’s Borgland Family Brain Tissue and DNA Bank, and ran them through a machine that sequences the entire coding genome of one person in a few days for a few hundred dollars – something that a decade ago would have taken years and millions of dollars.
Within three months of sifting through the resulting data, he found a rare mutation in two families – a substitution of one nucleotide for another in each affected person’s DNA. In those families, 70 per cent of the people with the mutation developed the disease.
Biological beacons
Dr. Vilarino-Guell then spent more than a year, working with Weihong Song, a UBC professor of psychiatry, to determine whether that mutation actually has a biological effect. If it didn’t, then it could have been viewed as a coincidence, with no clear link to MS.
But they confirmed that the mutation was indeed relevant: The mutation results in a defective protein that is unable to activate other genes that control inflammation. And uncontrolled inflammation around neurons – leading to a degradation of the brain cells’ protective coating – is what MS is all about.
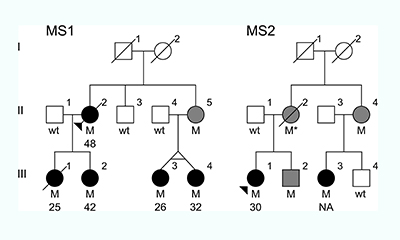
The family trees of the two unrelated families that had the MS-causing mutation. “M”=individuals with the mutation. Black circles=individuals with MS. Gray circles/squares=individuals with the mutation whose health is unknown.
These families were a beacon of sorts: Although the mutation is very rare, even in MS patients, its effects are so consistent and so glaring, they provide a window into the biological origins of the disease.
The researchers immediately embarked on the painstaking process of trying to find the families. Although each family had consented to being contacted by researchers, the initial study was long over, so the researchers had to get special permission to seek them out all these years later. Once that permission was granted, however, it turned out both families had “disappeared” – the treating physician no longer had them as patients, and had no idea of their or their relatives’ whereabouts.
“We were so disappointed, because it would have been exciting to analyze their brain MRI scans, and see if their disease looks different from other people with MS,” Dr. Vilarino-Guell says. “Or we could see if all of the people with the mutation eventually developed the disease or remained healthy – we don’t even know that. And even if they still don’t show any outward symptoms, that could provide clues as to how to stop the disease – what protected them, despite having the mutation?”
Reasons for hope
Nevertheless, the researchers are pumped.
The discovery of the mutation could enable the engineering of a more authentic animal model of MS – delivery of the first such mice is just weeks away. If mice with the mutation develop MS symptoms, they can be used to examine the cascade of biological reactions that lead to degradation of myelin, the protective coating of neurons. The mice could also be used for rapid testing of drugs before proceeding to more time-consuming and expensive human trials.
Another promising outcome of this discovery: The gene that contains this mutation is the site of more common mutations that appear in up to 50 per cent of people with the progressive form of MS. Most of those other mutations have been explored, and unlike the one found in the two families, don’t appear to have a direct impact on protein structure. But maybe, Dr. Vilarino-Guell speculates, those mutations somehow affect expression of the gene, thus reducing how much protein is produced.
“So if you can treat the families with this very rare mutation, you might be able to treat all of the other families that have progressive MS, even if they don’t have this mutation,” he says.
But unlike many genetic discoveries, this one might influence treatment very soon. People with a family history of MS could be screened for this mutation, and if they carry it, could be more closely monitored and perhaps start receiving treatment before outward symptoms appear.
Dr. Sadovnick, ever-so-guarded after decades of facing down skeptics, cautions that their results need to be replicated by others. But she concedes that this might represent a tipping point in recognizing that genes can play a major role in MS.
“My gut says there are probably families where it’s much more genetics, and some families where it’s more environmental, and other families where it’s almost equal,” she says. “Genes don’t operate in a vacuum – there are many things that influence them. But environment doesn’t operate in a vacuum, either. If you have the right combination, it can push you over the edge.”