Blood platelets have one main job: Stop bleeding by forming clots. Sometimes, however, these tiny cell fragments fail when they are needed most – when a person is experiencing massive bleeding, usually due to trauma.
A University of British Columbia bioengineer has developed a potential strategy for endowing platelets with extra powers so they can rise to the occasion and continue coagulation. If it’s proven to work in clinical situations, such “superplatelets” might become a standard part of emergency department supplies, along with bandages, oxygen and saline.
“Coagulation, which depends on a series of complex biochemical reactions, works great for scrapes and paper cuts,” said Christian Kastrup, an Associate Professor in the Department of Biochemistry and Molecular Biology. “But trauma often overwhelms this intricate, delicate process. We wanted to make it more resilient.”
Platelets are the first-responders to blood vessel ruptures – they swarm to the edges of an exposed vessel wall, and their shape changes from smooth and round to sticky and star-like, so they can easily clump together.
But that is only part of coagulation. To plug a wound, the platelets must be woven together into a spongy mass that hardens and contracts into a clot. That weaving is done by a tough material called fibrin, created through a multi-step chain reaction that depends on an enzyme called thrombin.
Under extreme stress, such as trauma, that chain reaction fizzles out for a variety of reasons – what doctors call “trauma-induced coagulopathy” or “TIC.”
Dr. Kastrup, a scientist in UBC’s Michael Smith Laboratories and the Centre for Blood Research, wondered if platelets could be modified to play a bigger role in coagulation, and effectively rescue the reaction.
As described in the Journal of Thrombosis and Haemostasis, Dr. Kastrup and graduate student Vivienne Chan inserted thrombin into natural, nano-sized, bubble-like containers called liposomes, and mixed them with platelets by the hundreds of thousands. After the platelets absorbed the nanoparticles, they immersed them in various types of blood samples.
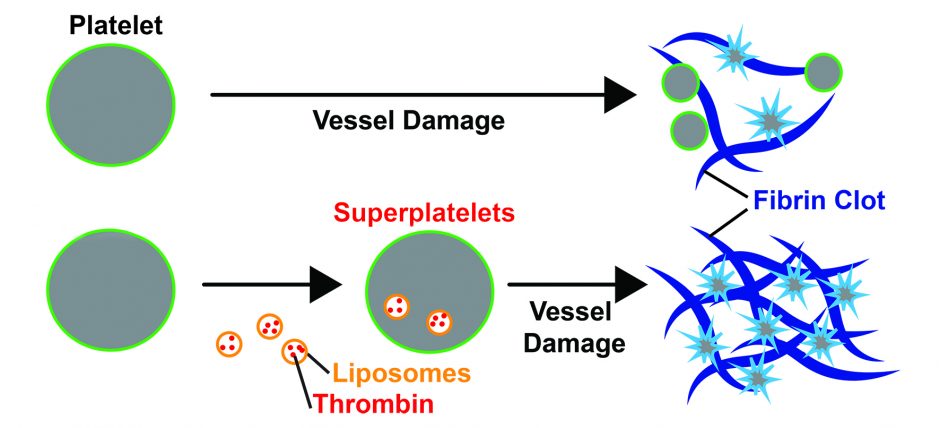 The thrombin-loaded platelets clotted blood from healthy people approximately 30 per cent faster compared than normal platelets, and formed clots that were 20 per cent stronger. In blood collected from two Seattle patients with TIC, the modified platelets clotted blood 20 per cent to 40 per faster.
The thrombin-loaded platelets clotted blood from healthy people approximately 30 per cent faster compared than normal platelets, and formed clots that were 20 per cent stronger. In blood collected from two Seattle patients with TIC, the modified platelets clotted blood 20 per cent to 40 per faster.
Modified platelets also fully compensated for the delayed clotting times caused by elevated blood acidity (a common occurrence in trauma) or by aspirin and naproxen, drugs often taken regularly by people specifically for their anti-clotting properties as a way of preventing heart attack or stroke. They also performed well in blood taken from people with hemophilia, a disease that prevents the blood from clotting.
If these superplatelets work in animal models, in human clinical trials and are ultimately approved for medical use, Dr. Kastrup envisions that trauma centres could have bags of modified plasma on hand for patients with severe bleeding. The modification could conceivably be done at the time that donated blood is processed.
“Trauma is the leading killer of people under 45 years old, and blood loss is the second most common cause of such deaths,” Dr. Kastrup said. “By tweaking the body’s own clotting mechanism, we might be able to make trauma more survivable.”
